For cool and safe experiments sign up to MEL Science here: https://goo.gl/tRuRMd Did you think that ascorbic acid was just a tasty vitamin? It’s also a powerful antioxidant and reagent which can be used for impressive experiments! In this experiment you will learn about the main properties of ascorbic acid, and find out why it is so important for health. Reagents and equipment: * 3% hydrogen peroxide solution; * 10% iron(II) sulfate solution; * 10% ammonium thiocyanate solution; * 1% ascorbic acid solution; * lemon juice; * pipette; * beaker. Step-by-step instructions Into three beakers, pour an iron(II) sulfate solution. Add lemon juice to the first beaker, leave the second unchanged, and to the third, add a solution of ascorbic acid. Now pour the solutions of ammonium thiocyanate and hydrogen peroxide into each beaker. Observe the color change to red in the second beaker. Processes description Antioxidants are substance which block oxidation reactions in the body. One of these substances is ascorbic acid. It is contained in many fruit and vegetables, for example in citrus fruit and red bell pepper. In the first and third beaker, ascorbic acid blocks the oxidation of iron(II) to iron(III), i.e. it absorbs the oxygen radicals which form in the breakdown of hydrogen peroxide. There is no ascorbic acid in the second beaker, and we observe the red bond of iron (III) thyocyanate form Fe²⁺ + 2H₂O₂ + 3CNS⁻ → Fe(CNS)₃ + 2H₂O + O₂

Why Vitamin C is useful (“Ascorbic acid: a strong antioxidant” experiment)
- Post author:admin
- Post published:October 4, 2021
- Post category:Uncategorized
- Post comments:0 Comments
You Might Also Like
Cholesterol Blood Test

Aromatic Oils Video – 1

Gynecomastia Treatment Dallas – Overcome Gynaecomastia Promptly

Anabolic Steroids – History, Definition, Use & Abuse Video – 27

Digestive System And Asnas Video – 2

Close Grip Triceps Extension-5

The Best Protein Powders – Our Favorite Whey Options (NOW!)

Minerals- What is a Mineral

The Best Science-Based Leg Workout for Growth (Glutes/Quads/Hams)

Antioxidant – Antioxidant Foods

How to Use Whey Protein for Weight Loss

High Intensity Training Video – 5

Belly Fat
Acupuncture/Acupressure Video – 4

Face mapping: What is your acne telling you?
Surgery for Hypertrophic Cardiomyopathy
CT Angio Brain

Olive Oil Recipes To Lose Weight

Side plank-5

What Is Cholesterol

Goalkeeper Training: Game Warm up

Bent over lateral raises
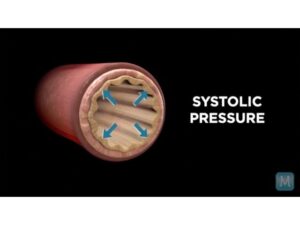
Systole vs. Diastole | Match Health

HGH, Growth Hormones & Plant Hormones Video – 43

Male Breast Reduction Surgery In Trivandrum | Gynecomastia Surgery | Gynecomastia Treatment Kerala

Arnold Press – Shoulder Exercise – Proper Form Tutorial
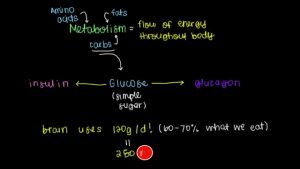
Insulin and Glucagon
Orthopedic Physiotherapy Video – 1

Amino Acid Therapy for Mental Health + Addictions
Shrugs-5

Sugar Free, Low Sugar Video – 5

Joe Rogan and Mark Sisson on Post Workout Shakes

FAQ 11: What To Eat Before and After a Workout | Health and Fitness
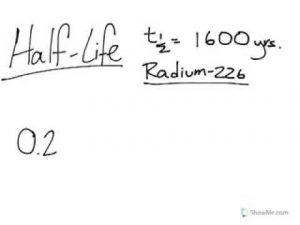
Half Life Calculations Part 1 (easy way)
How do you calculate heart rate from ECG?

Ask Torre – Session 1

Dumbbell Bent-Over Row Single Arm. Bodybuilding Exercise Anatomy Animation Stock Video buy online.

How To: Barbell Upright Row

Fat Loss Foods Video – 1

Making A Yoga Routine Video – 7

Abscess – Causes, Treatments & More…

