In this video we cover the structure of fatty acids and the different types of fatty acids. Fatty acids are made up of long chains of carbon atoms and hydrogen atoms. Some carbon atoms are linked by single bonds, and others by double bonds. These bonds determine which type of fatty acid the molecule is classified as. There are 2 types of fatty acids, saturated fatty acids, and unsaturated fatty acids, which include monounsaturated fatty acids, polyunsaturated fatty acids and trans fatty acids. In saturated fatty acids, all of the carbon atoms are saturated with hydrogen atoms and do not contain double bonds between the carbon atoms, this gives the molecule a linear formation. Saturated fats are usually solid at room temperature, and have high melting points. Foods that are high in saturated fat include pork, fatty beef, cheese, whole milk, eggs, coconut and palm oils and butter. Unlike saturated fatty acids, unsaturated fatty acids have at least one double bonded set of carbon atoms in their structure. This double bond can take on one of 2 formations. It can be a cis configuration or a trans configuration. In the cis formation, the hydrogen atoms are on the same side of the double bonded carbon atoms, and in the trans formation, the hydrogen atoms are on opposite sides. Trans fats are solid at room temperature and usually have a high melting point. There are natural and artificial trans fats. Natural trans fats, also known as ruminant trans fats, typically make up 2 to 5% of the fat in dairy products and 3 to 9% of the fat in beef and lamb. Artificial trans fats are formed when manufacturers turn liquid oils into solid fats through a process called hydrogenation. Hydrogenation is a process by which vegetable oils are converted to solid fats simply by adding hydrogen atoms. Some foods that contain trans fats include stick margarine, fried foods and many fast food items. Monounsaturated fatty acids have a cis molecular formation, where the hydrogen atoms are on the same side of the double bonded carbon atoms, this gives it a bend, or a kinked like formation. Monounsaturated fats have only one carbon carbon double bond in their molecule. They are usually liquid at room temperature and have lower melting points than saturated and trans fats. Foods that are high in monounsaturated fat include many plant based oils such as olive oil, canola oil and peanut oil. Polyunsaturated fatty acids also have a cis molecular formation. Again, the hydrogen atoms are on the same side, of the double bonded carbon atoms also giving it a kinked formation. Polyunsaturated fats have more than one unsaturated carbon double bond in their molecule. They are typically liquid at room temperature, but start to turn solid when chilled. Polyunsaturated fats are generally classified by their Omega numbering. The omega carbon is the carbon atom at the end of the hydrocarbon chain. There are 4 types of omega fatty acids, 3, 6, 7, and 9. These are determined by where the location of the 1st double bonded carbon atom is located. The fatty acid on the screen is an omega 3 fatty acid, because the 1st double bond occurs at carbon number 3. The other omega fatty acids follow this same structure.
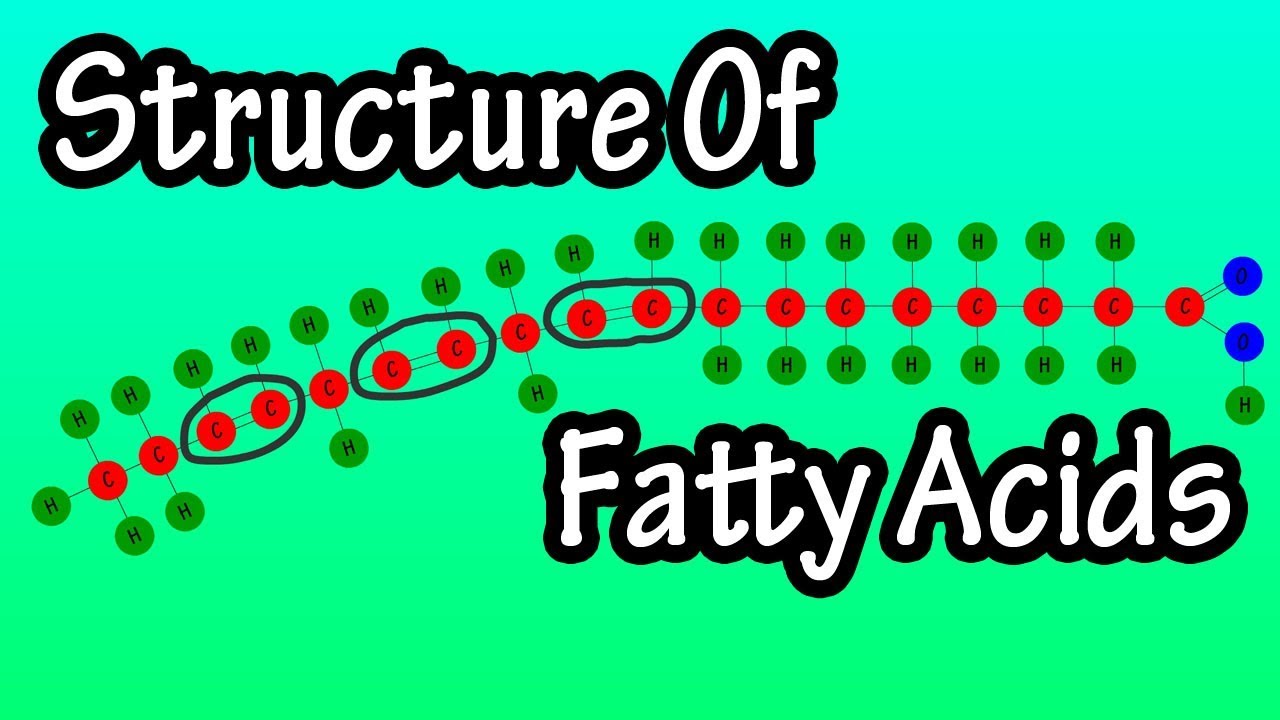
Fatty Acids – What Are Fatty Acids – Structure Of Fatty Acids – Types Of Fatty Acids
- Post author:
- Post published:May 15, 2021
- Post category:Uncategorized
- Post comments:0 Comments
You Might Also Like

Donkey Kicks-10

Human Body Systems Video – 4

Fitness Component of Flexibility

Anabolic Steroids – History, Definition, Use & Abuse Video – 39

What is Fish Oil? Omega-3 Benefits & Side Effects Review by Guru Mann

Intermittent Fasting & Fasting Video – 10
Epidemiology Video – 2

Muscle Building Workout & Squats Video – 12

Wrestling Video – 3

Fitness Definition Components Video – 5
Acne | Nucleus Health

How to do a preacher curl for maximum results!!

Branches of Physiotherapy Video – 26

How to do a Barbell Row- Fitness Zone at intosport.com

How Much Fruit is Too Much Fruit?

Kriya Video – 6

Yoga Diet Video – 3

Benefits Of Human Growth Hormone Releasers

Fat Loss, Weight Loss Video – 24

The Ultimate Thyroid Home Test with Dr. Rob

Babyface Bizzy – B.M.R

AEROBIC vs ANAEROBIC DIFFERENCE

Protein Powder | Dr. Shel Wellness & Aesthetic Center
Elbow flexion, shoulder flexion, and forearm supination (Biceps brachii) muscle actions

How to Do a Side Plank | Ab Workout

Exercises to Define the Waist : Dynamic Exercises
![Read more about the article Greenatr weight loss kit [animations video portfolio]](https://videos.drmaheshkumar.com/wp-content/uploads/2021/06/Greenatr-weight-loss-kit-animations-video-portfolio-300x169.jpg)
Greenatr weight loss kit [animations video portfolio]

Jogging on Spot
Floatation Healing Video – 4

Understanding semen analysis – Dr. Teena S Thomas

Keto Diet, Keto Foods, Keto Recipes Video – 13
Laproscopic Surgeries Video – 1

Female Body Types And Body Shapes Different Body Types Women Have

Digestion Song

Stability Ball Hyperextension – HASfit Low Back Exercises – Lower Back Exercise

Carbohydrates – Types Of Carbohydrates – What Are Carbohydrates – What Are Good Carbs And Bad Carbs
Diazepam – Mechanism of Action

What is Fish Oil? Omega-3 Benefits & Side Effects Review by Guru Mann

Incline Bench Press-1

Bodybuilding Video – 3

